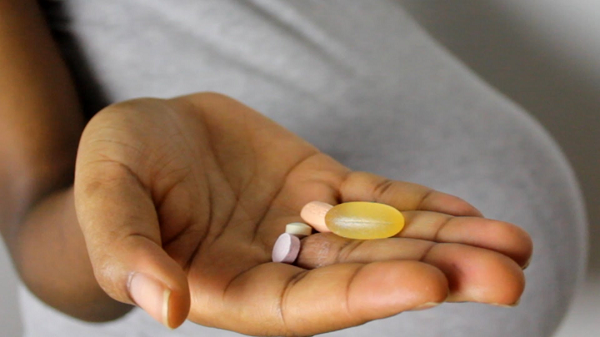
Ghana has been hit by a growing trend of pregnant women taking the skin-lightening pill glutathione in the past few months. The women hope to make their unborn babies light-skinned while still in the womb.
According to BBC and B&FT Online, Ghana’s Food and Drugs Authority (FDA) has warned women to stop using the pill, saying it is not FDA-approved and therefore illegal.
They stress that using these pills to lighten the skin of unborn babies is dangerous.
The tablets are smuggled into the country via airline-passenger luggage in large quantities – despite medical experts warning that they can cause side effects in a foetus, which can include physical damage to limbs.
“The use of these drugs has gone to an alarming stage; it is ignorance that is making people do so,” the FDA’s head of cosmetics and household chemicals, Emmanuel Nkrumah, said at a media-sensitisation workshop on unapproved bleaching pills and products.
“[The only things] that you take orally should be food, toothpaste and mouthwash, and not bleaching pills,” Nkrumah said.
The FDA says using glutathione pills for this purpose is dangerous, adding that it wants “the general public to know that no product has been approved by the FDA in the form of a tablet to lighten the skin of the unborn child”.
Glutathione skin lightening has been gaining popularity in different countries.
Here are facts about the side effects of glutathione according to Light Skin Cure, a skincare-oriented website:
- Allergic reactions – Some people may experience skin swelling or rashes on parts of the body.
- Cancer-causing ingredients – Research indicates that some ingredients in most skin-whitening products, glutathione included, may cause cancer.
- Disruption of normal skin functions – Glutathione may interfere with the normal production of melanin in skin. Some ingredients could also affect the body’s hormone levels.