NAFDAC warns against use of ‘carcinogenic’ weight loss drug
The National Agency for Food and Drugs Administration and Control (NAFDAC) has warned Nigerians against the use of a drug — Weight Rapid Loss Capsule.
Mojisola Adeyeye, NAFDAC director-general, in a statement, said the drug has been found to have the potential to cause cancer.
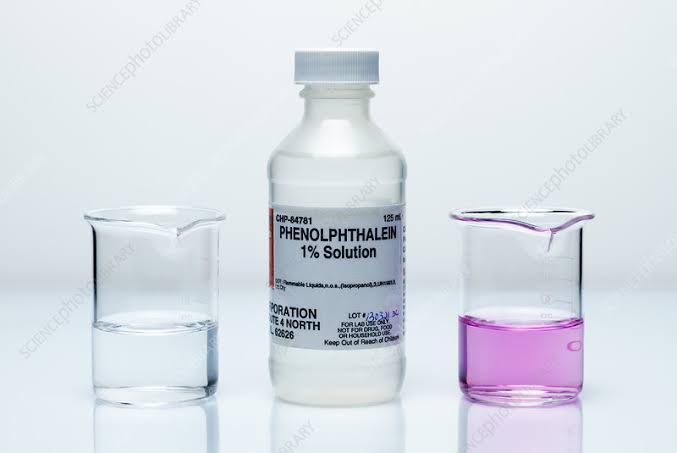
Adeyeye said results of laboratory analysis showed that the drug, manufactured by Ingi Oman, contained a banned substance called “phenolphthalein”, which the US Food and Drug Administration recognised as unsafe.
She said the capsule, being marketed as “the most effective weight loss supplement” and sold through social media platforms like Instagram, also contains microbial growth above permissible limits.
“Phenolphthalein has been found to be toxic to genes, as it can cause damage or mutations in the DNA. Studies have also shown its potential carcinogenic risks,” the statement reads.
“NAFDAC implores consumers to stop the purchase and use of the product.
“Members of the public in possession of the product should discontinue use or sale, and submit stock to the nearest NAFDAC office.”
Adeyeye encouraged healthcare professionals and consumers to report any adverse effect experienced with the use of the product to the nearest NAFDAC office.
“Consumers are also advised to report adverse effects via pharmacovigilance@nafdac.gov.ng or E-reporting platforms available at www.nafdac.gov.ng,” the statement reads.