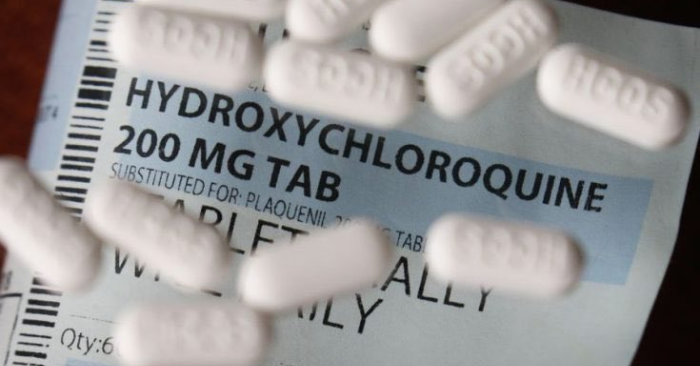
According to new research published in the preprint server medRxiv in July 2020, the use of hydroxychloroquine (HCQ) among outpatients in clinical trials, without high-risk factors for cardiac arrhythmia, is safe.
With gastrointestinal side-effects being the most common side effects and no fatal adverse outcomes.
Hydroxychloroquine is a drug with anti-inflammatory properties commonly used to treat malaria, rheumatoid arthritis, and systemic lupus erythematosus.
READ ALSO: WHO suspends trial of hydroxychloroquine over safety concerns
As the COVID-19 pandemic continues all around the world, with over 630,000 deaths so far, scientific research has focused on finding effective medications against the virus. A strong contender, right from the very beginning, has been HCQ, mainly because of the heavy backing given by political heavyweights.
HCQ has been demonstrated to have in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19 disease, and hinders its replication. However, correspondingly strong evidence of its activity in the treatment or prevention of this disease has not been found so far.
For this reason, the World Health Organization and several other health organizations are conducting studies on the safety and efficacy of this drug in both asymptomatic or mildly symptomatic cases, as well as sicker patients in hospitals.
Earlier research has reported an increase in the reported incidence of cardiac side effects with the use of HCQ and azithromycin in combination. This led to the revocation of the Emergency Authorization issued by the US Food and Drug Authority (FDA) for the use of HCQ in severely ill COVID-19 patients.